- Triple-negative breast cancer (TNBC) is more aggressive than other breast cancer subtypes, with limited treatment options and poor prognosis
- Newly discovered method tames the behaviour of aggressive TNBC cells, rendering them more responsive to chemotherapy
- Findings have kick-started a clinical trial to investigate role of retinoids in treating TNBC
Singapore, 7 October 2021 – A team of clinicians and scientists from the National Cancer Centre Singapore (NCCS), Singapore General Hospital (SGH) and A*STAR’s Genome Institute of Singapore (GIS) has identified a novel method to treat triple-negative breast cancer (TNBC). They discovered that cancer cells switch between different cell states and are able to change from being less aggressive (‘epithelial’) to being more aggressive (‘mesenchymal’), and vice versa. By converting highly aggressive cancer cells to become less aggressive, tumours are primed to respond better to chemotherapy, which works to eliminate cancer cells. This discovery has led to the launch of a three-year long human clinical trial, BEXMET (Bexarotene-induced Mesenchymal-Epithelial Transition), to investigate this unconventional approach to treating TNBC.
TNBC is more aggressive than other breast cancer sub-types, with limited treatment options and a poor prognosis. TNBC tests negative for estrogen receptor (ER), progesterone receptor (PR) and human epithelial growth factor receptor-2 (HER2), hence the reference to ‘triple-negative’ in its name. This also means that treatments targeting ER, PR and HER2 are not effective. For that reason, chemotherapy is still the mainstay standard treatment for TNBC.
The development of novel oncology drugs is costly, and affordability and accessibility can be a challenge. The concept tested by the NCCS, SGH and GIS team involves altering cancer cell states so that they are more susceptible to currently available chemotherapy. This may be a cost-effective way to treat TNBC, with the potential to treat a wider range of other cancers.
The team began by studying breast cancer tissue from NCCS and SGH patients in 2017 to understand pathways that control the behaviour of cancer cells, in terms of their ability to invade and spread. Leveraging highly advanced genome sequencing and functional genomic capabilities at GIS, the research found that Bexarotene – belonging to a class of drugs known as retinoids – is able to convert the more aggressive ‘mesenchymal’ cell state to a less aggressive ‘epithelial’ cell state. This biological process is also termed mesenchymal-to-epithelial transition (MET). This is the first time that Bexarotene has been employed to facilitate the MET process in preclinical breast cancer work.
These findings were published in the journal Science Advances in October 2021¹. The study was led by co-senior author, Dr Tam Wai Leong, Associate Director and Group Leader of the Laboratory of Translational Cancer Biology at GIS.
“Cancer cells are crafty and have ways to evade treatment, sometimes through taking on this drug-resistant ‘mesenchymal’ cell state. Instead of tackling the tumours conventionally with direct administration of chemotherapy, the solution may be to first coax them into a less aggressive state before chemotherapy is given. This is a departure from standard cancer treatment,” said Dr Tam. “Taming the behaviour of such malignant cells prior to their ablation may work better and result in more durable clinical responses.”
In preclinical tests performed in animal models, Bexarotene changed TNBC cell states from mesenchymal to epithelial, rendering the TNBC cells more susceptible to conventional chemotherapy, which resulted in longer lasting responses that kept cancer relapse at bay.
These laboratory findings were directly translated to the human setting with the clinical trial, BEXMET. The trial aims to validate Bexarotene-induced TNBC cell state changes that were observed pre-clinically, and assess the tolerability and efficacy of the Bexarotene-Capecitabine chemotherapy combination².
“Laboratory-based findings published in a scientific journal don’t always translate to the clinical setting for various reasons. For our study, there is an existing clinical-grade version of the MET inducer (Bexarotene), which significantly facilitated the direct translation to the clinical setting. We hope that results from BEXMET will be the first step in introducing a novel concept in cancer treatment,” said co-senior author and BEXMET principal investigator Dr Elaine Lim, Senior Consultant, Department of Breast and Gynaecology, Division of Medical Oncology, NCCS.
Deputy Medical Director of Research at NCCS, Professor Teh Bin Tean shared, “The BEXMET clinical trial is a testament to the strong bench-to-bedside research pipeline that the National Cancer Centre Singapore has built, in collaboration with partners like A*STAR’s Genome Institute of Singapore. We are perfectly poised to apply basic science research to address the needs of cancer patients and improve health outcomes.”
BEXMET is supported by the National Research Foundation (NRF) under the Clinical Trials Grant–Investigator-Initiated Trials Scheme (MOH-000581) and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC). Pre-clinical work was funded by A*STAR, the NMRC, and the NCC Research Fund. This bench-to-bedside research is conducted under the auspices of Breast Cancer Research Singapore, a consortium of clinicians and scientists dedicated to breast cancer research in Singapore, under the patronage of President Halimah Yacob.
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
References:
-
Loo, S. Y. et al. Fatty acid oxidation is a druggable gateway regulating cellular plasticity for driving metastasis in breast cancer. Science advances 7 : eabh2443 (2021).
- The Role of Bexarotene in Inducing Susceptibility to Chemotherapy in Metastatic TNBC. ClinicalTrials.gov Identifier: NCT04664829 https://clinicaltrials.gov/ct2/show/NCT04664829.
Illustrations:
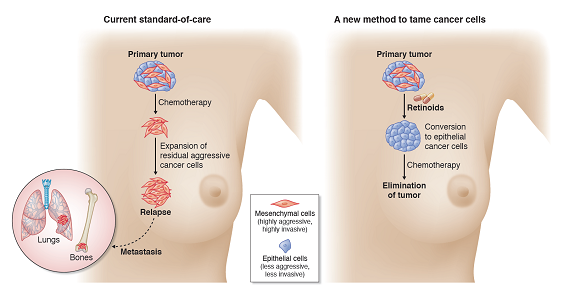
Figure 1. Research findings suggest that neoadjuvant treatment with retinoids may promote the conversion of highly aggressive mesenchymal cancer cells to less aggressive epithelial cancer cells in breast cancer. This can render breast tumours more responsive to subsequent chemotherapy and produce a durable treatment outcome. A clinical trial is underway to examine this new concept of treatment.
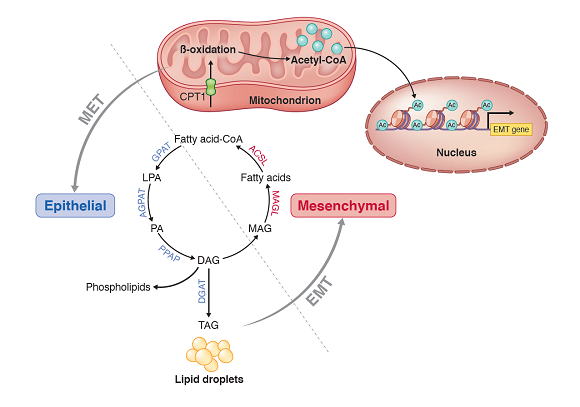
Figure 2. Scientific model illustrating the different use of fatty acid in specific cancer cell states. In the more aggressive mesenchymal cell state, beta-oxidation is activated to burn fatty acid; this generates acetyl-CoA – a bioactive metabolite – for the epigenetic control of genes responsible for the epithelial-mesenchymal transition and cancer metastasis. Upon the mesenchymal-to-epithelial transition, which is induced by retinoid treatment, fatty acid is stored as lipids instead. By preventing the ability of cancer cells to oxidise fatty acid, the less aggressive epithelial cancer cells are unable to acquire aggressive behaviour and less likely to metastasise.
Photo:

Caption: Team members leading the preclinical study and the BEXMET clinical trial. (Left) Dr Loo Ser Yue, Senior Research Fellow, Genome Institute of Singapore, A*STAR; (Middle) Dr Elaine Lim, Senior Consultant, National Cancer Centre Singapore; (Right) Dr Tam Wai Leong, Group Leader, Genome Institute of Singapore, A*STAR.
For more information, please contact:
National Cancer Centre Singapore
Dharshini Subbiah
Assistant Manager, Corporate Communications
Email : [email protected]
Genome Institute of Singapore (GIS)
Lyn Lai
Office of Corporate Communications
Email: [email protected]
About the National Cancer Centre Singapore
The National Cancer Centre Singapore (NCCS) provides a holistic and multi-disciplinary approach to cancer treatment and patient care. We see close to 65 per cent of the public sector oncology cases, and they are benefiting from the sub-specialisation of our clinical oncologists.
To deliver among the best in cancer treatment and care, our clinicians work closely with our scientists who conduct robust, cutting-edge clinical and translational research programmes which are internationally recognised. NCCS strives to be a global leading cancer centre, and shares its expertise and knowledge by offering training to local and overseas medical professionals.
For more information, please visit: www.nccs.com.sg
About the Genome Institute of Singapore (GIS)
The Genome Institute of Singapore (GIS) is an institute of the Agency for Science, Technology and Research (A*STAR). It has a global vision that seeks to use genomic sciences to achieve extraordinary improvements in human health and public prosperity. Established in 2000 as a centre for genomic discovery, the GIS pursues the integration of technology, genetics and biology towards academic, economic and societal impact, with a mission to "read, reveal and write DNA for a better Singapore and world".
Key research areas at the GIS include Precision Medicine & Population Genomics, Genome Informatics, Spatial & Single Cell Systems, Epigenetic & Epitranscriptomic Regulation, Genome Architecture & Design, and Sequencing Platforms. The genomics infrastructure at the GIS is also utilised to train new scientific talent, to function as a bridge for academic and industrial research, and to explore scientific questions of high impact.
For more information about GIS, please visit www.a-star.edu.sg/gis.
About the Agency for Science, Technology and Research (A*STAR)
A*STAR is Singapore's lead public sector R&D agency. Through open innovation, we collaborate with our partners in both the public and private sectors to benefit the economy and society. As a Science and Technology Organisation, A*STAR bridges the gap between academia and industry. Our research creates economic growth and jobs for Singapore, and enhances lives by improving societal outcomes in healthcare, urban living, and sustainability. A*STAR plays a key role in nurturing scientific talent and leaders for the wider research community and industry. A*STAR’s R&D activities span biomedical sciences to physical sciences and engineering, with research entities primarily located in Biopolis and Fusionopolis. For ongoing news, visit this website.
Follow us on
Facebook | LinkedIn | Instagram | YouTube
