Pharmacogenomics is a powerful tool in precision medicine that GPs can harness to guide optimised, personalised care for patients – with the potential to improve safety and efficacy. Find out more.
Pharmacogenomics is a powerful tool in precision medicine that general practitioners can harness to guide optimised, personalised care for patients – with the potential to improve safety and efficacy. The SingHealth Duke-NUS Genomic Medicine Centre shares about its latest clinical applications and initiatives relevant to primary care.
WHAT IS PHARMACOGENOMICS?
The advent of precision medicine holds promise to transform healthcare through early disease detection, refining diagnoses and tailoring treatment. Pharmacogenomics, an essential branch of precision medicine, is the study of how genetic variations influence the body’s response to medications.1
Genetic variations may affect drug response by changing the expression or activity of genes that encode enzymes or molecular targets that are involved in the pharmacokinetic or pharmacodynamic pathways.
Established data
Currently, there are multiple drug-gene pairs with established evidence and actionable prescribing guidance. Clinical information pertaining to the drugs and genes of interest has been curated by expert bodies such as the Pharmacogenomics Knowledge Base (PharmGKB) and the Clinical Pharmacogenetics Implementation Consortium (CPIC).1,2
Local data from the SG10K_Health study showed that 99.7% of the cohort carried at least one actionable pharmacogenomics variant out of 23 pharmacogenes with high-confidence gene-drug associations, with a median of five variants per individual.3 Notably:
A significant proportion of individuals carry actionable pharmacogenetic variants for CYP2C19 (61.8%) and CYP2D6 (43.0%), which are important in the metabolism of commonly used drugs such as clopidogrel, antidepressants and opioids.
Approximately one-quarter (25.6%) of individuals harboured a genotype (HLA-A or HLA-B risk allele) associated with increased risk of allopurinol- or carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).
Taken together, pharmacogenomics may be utilised for the optimisation of pharmacotherapy, allowing a personalised approach that has the potential to improve safety and efficacy.
Examples of Clinical Applications of Pharmacogenomics
1. Carbamazepine – HLA-B*15:02
Carbamazepine is an antiseizure drug indicated for the treatment of epilepsy, trigeminal neuralgia and bipolar disorders.
Research
Local studies show that there is a strong association between the HLA-B*15:02 allele and carbamazepine-induced SJS/TEN and testing for the allele is cost-effective.4,5
Applications
In April 2013, the Ministry of Health (MOH) announced that genotyping for the HLA-B*15:02 allele prior to initiating carbamazepine therapy in new patients of Asian ancestry is considered the standard of care in Singapore.6 All subsidised patients at public healthcare institutions are eligible for a 75% subsidy for HLA-B*15:02 testing.
Following this regulatory recommendation by MOH and the Health Sciences Authority (HSA), a 92% reduction in cases of SJS/TEN that are associated with carbamazepine was observed within five years.7
Key points to note
For new patients of Asian ancestry, pharmacogenetic testing of HLA-B*15:02 is recommended prior to initiating carbamazepine therapy (standard of care). If the result is:
HLA-B*15:02 positive: An alternative agent is recommended. Phenytoin should be avoided as there is an increased risk of SJS/TENS.
HLA-B*15:02 negative: Carbamazepine can be initiated, but continue to monitor for the first three months. Clinical vigilance for carbamazepine-induced severe cutaneous adverse reactions (SCARs) including drug reaction with eosinophilia and systemic symptoms (DRESS) should be continued, as there may be other non-genetic factors (e.g., concomitant drugs and comorbidities) that have not been studied.
For patients who have taken carbamazepine for more than three months but are found to be HLA-B*15:02 positive:
2. Allopurinol – HLA-B*58:01
Allopurinol is a commonly prescribed drug for the management of gout in the primary care setting. Though generally well-tolerated, allopurinol is associated with SCARs that may manifest as DRESS, SJS and TEN.8
Research
The presence of the HLA-B*58:01 allele has been associated with significantly increased risk of allopurinol-induced SCARs.9
Applications
Patients who have tested positive for HLA-B*58:01 are recommended to avoid starting allopurinol and consider alternative agents such as febuxostat.
In March 2016, HSA and MOH issued a communication to inform that HLA-B*58:01 genotyping prior to the initiation of allopurinol is not required as standard of care. This recommendation was made in view of the low positive predictive value (2%) and the lack of cost-effective alternatives for urate-lowering therapies.10
A reminder was issued by HSA in 2021 to advise healthcare professionals to consider HLA-B*58:01 genotyping in patients starting on allopurinol who have other pre-existing risk factors for allopurinol-induced SCARs – such as renal impairment and advanced age.11
Local clinical guidelines for gout management reiterated this position on HLA-B*58:01 testing and highlighted other non-genetic risk factors such as concurrent diuretic use, high starting allopurinol dose and rapid dose escalation.12
Key points to note
The presence of HLA-B*58:01 allele is associated with significantly increased risk of allopurinol-induced SCARs – allopurinol therapy should not be initiated.
Routine testing prior to starting allopurinol is not required as standard of care, though this may be considered for patients with pre-existing renal risk factors such as renal impairment and advanced age.
Consider other non-genetic risk factors of allopurinol-induced SCARs and adopt ‘start low, go slow’ dosing strategy.
Educate patients to recognise SCAR symptoms and seek medical attention promptly.
3. Clopidogrel – CYP2C19
Clopidogrel is a commonly prescribed antiplatelet that is widely used in coronary artery disease, stroke and peripheral arterial disease. It is a thienopyridine prodrug that is metabolised primarily by CYP2C19 into an active metabolite that is responsible for the antiplatelet action.
Research
An extensive number of studies have demonstrated that patients who carry the loss-of-function (LOF) alleles have a significant decrease in active metabolites and are at increased risk of ischaemic events when treated with clopidogrel.13
Applications
A recent American Heart Association (AHA) scientific statement proposed an algorithm using CYP2C19 pharmacogenetic testing to guide selection of P2Y12 inhibitor therapy in patients with coronary artery disease.14 Currently, there is no local guideline that addresses the use of CYP2C19 genotyping, and routine testing is not considered standard of care.
For neurovascular indications, clinical evidence from the CHANCE-2 study15 and local cost-effectiveness data16 support the use of CYP2C19 testing to guide selection of antiplatelets in patients with transient ischaemic attack (TIA) or mild stroke. Current recommendations from international guidelines propose switching to alternative antiplatelets in patients who are CYP2C19 poor or intermediate metabolisers.13
Key points to note
Routine testing of CYP2C19 before clopidogrel is not considered standard of care.
When CYP2C19 results are available, patients who are LOF allele carriers (poor or intermediate metabolisers) are recommended to switch to alternative antiplatelets. Aspirin, aspirin plus dipyridamole or ticagrelor are possible alternatives for stroke prevention.
Other non-genetic factors may influence the choice of antiplatelets – if in doubt, consult specialists for indication-specific choices.
Pharmacogenomics Initiatives Arising from the National Precision Medicine Strategy: What GPs Should Know
The National Precision Medicine (NPM) strategy is a 10-year plan that aims to enhance and accelerate Singapore’s biomedical research, health outcomes and economic growth. NPM is a whole-of-government effort to establish the necessary frameworks and infrastructure to realise precision medicine on a national scale – to ultimately improve public health, enhance disease prevention and identify the right treatments for the right individuals and groups.17
Below are examples of some initiatives related to pharmacogenomics that may be relevant to general practitioners (GPs).
1. Moratorium on Genetic Tests and Insurance
Pharmacogenomics tests are considered as predictive genetic tests and are covered under the 'Moratorium on Genetic Testing and Insurance.'18
Developed by MOH and the Life Insurance Association (LIA), the Moratorium aims to prevent individuals from being deterred to undergo clinical genetic tests for any medical indications and/or participating in precision medicine research due to concerns about insurability.
GPs may refer to this document for guidance should patients have concerns pertaining to insurability when pharmacogenomics tests are ordered.
2. Clinical Implementation Pilot: Pre-emptive Pharmacogenomic Testing in Routine Clinical Practice
Precision Health Research, Singapore (PRECISE) is a central entity set up to drive Phase II of the NPM strategy.
As part of the NPM Phase II, PRECISE provides funding for Clinical Implementation Pilots (CIP) that facilitate the incorporation of genetic/genomic tests into clinical pathways which improve patient outcomes while maintaining cost-effectiveness and sustainability.
One of the ongoing CIPs explores the use of pre-emptive multi-drug/multi-gene pharmacogenomic testing as a precision medicine tool in routine clinical practice
Pharmacogenomic panel testing results and recommendations for drugs in the panel will be accessible to patients on a digital platform in collaboration with a local startup. This will enable the sharing of pharmacogenomic information with clinicians from different institutions, including GPs.20
Results from this study will inform cost-effectiveness and implementation barriers that will be important for future care model design.
3. Code of Practice – Clinical and Clinical Laboratory Genetic/Genomic Testing Services
MOH has issued guidance on the requirementsnand standards for provision of clinical and clinicalnlaboratory standards for genomic/geneticnservices in the 'Updates to Code of Practicenon the Standards for the Provision of ClinicalnGenetic/Genomic Testing Services and ClinicalnLaboratory Genetic/Genomic Testing Services'.19
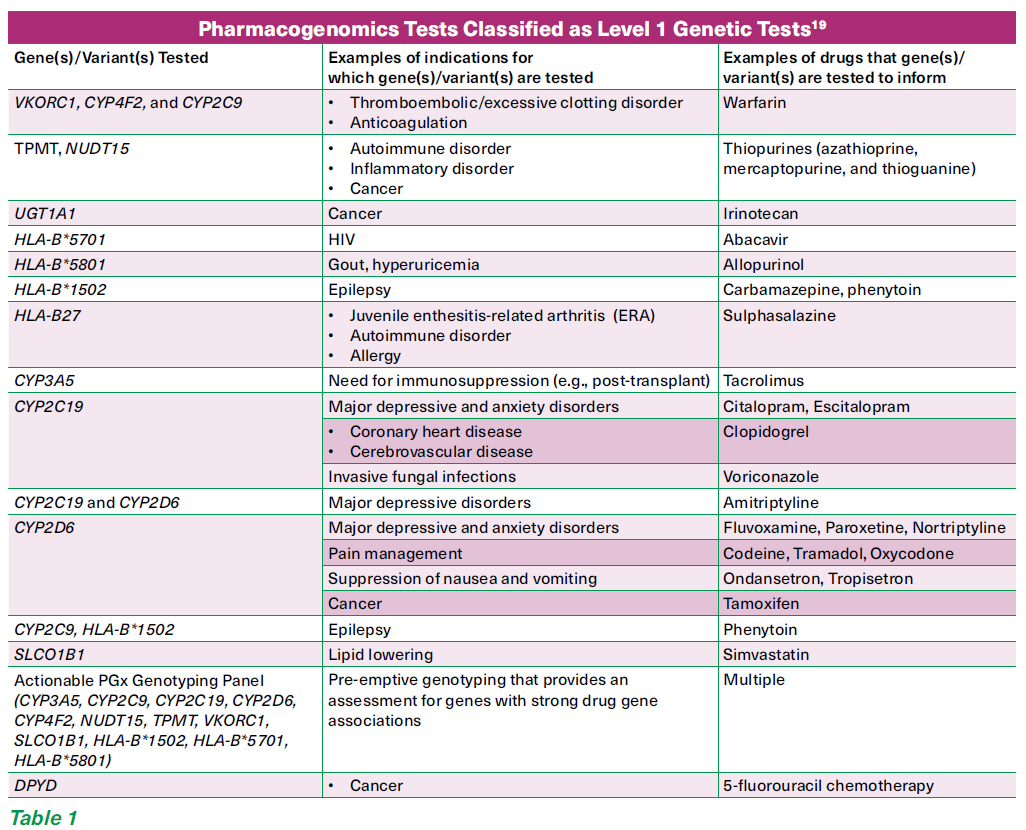
The Code of Practice stratifies genetic tests into three levels. The majority of the pharmacogenomics tests are classified under Level 1 (See Table 1)19 where:
The tests are deemed to be likely appropriately ordered and correctly interpreted by most registered medical practitioners.
Most medical practitioners are likely to be able to appropriately explain the test results to patients.
Most registered medical practitioners are likely to be able to implement the appropriate referrals, investigations and/or follow-up plans based on the test results.
For Level 1 genetic tests, pre-test and post-test genetic counselling is not applicable. However, pre/post-test counselling as per other diagnostic procedures is advised.
CONCLUSION
With the launch of the Healthier SG initiative in July 2023, GPs are playing an increasingly important role in providing preventive and chronic care. The challenges associated with multimorbidity and polypharmacy are to be anticipated as the nation is set to attain ‘super-aged’ status in 2026. Pharmacogenomics is a viable tool for personalised pharmacotherapy in the primary care setting. Innovative care models through the Primary Care Networks (PCN) may be explored to better support GPs in pharmacotherapy optimising and medication management.
REFERENCES
Whirl-Carrillo M, Huddart R, Gong L, et al. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin Pharmacol Ther. 2021 Sep;110(3):563-572. doi: 10.1002/cpt.2350. Epub 2021 Jul 22.
Clinical Pharmacogenetics Implementation Consortium (CPIC). Available from http://cpicpgx.org/
Chan SH, Bylstra Y, Teo JX, et al. Analysis of clinically relevant variants from ancestrally diverse Asian genomes. Nat Commun. 2022 Nov 5;13(1):6694. doi: 10.1038/s41467-022-34116-9.
Toh DS, Tan LL, Aw DC, et al. Building pharmacogenetics into a pharmacovigilance program in Singapore: using serious skin rash as a pilot study. Pharmacogenomics J. 2014 Aug;14(4):316-21. doi:10.1038/tpj.2013.46. Epub 2014 Jan 7.
Dong D, Sung C, Finkelstein EA. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology. 2012 Sep 18;79(12):1259-67. doi: 10.1212/WNL.0b013e31826aac73. Epub 2012 Sep 5.
Recommendations for HLA-B*1502 genotype testing prior to initiation of carbamazepine in new patients. Singapore: Health Sciences Authority; 2013 [updated: 29 Aug 2013; accessed: 15 Sept 2024]. Available from: https://www.hsa.gov.sg/announcements/safety-alert/recommendations-for-hla-b-1502-genotype-testingprior-to-initiation-of-carbamazepine-in-new-patients (hsa.gov.sg)
Sung C, Tan L, Limenta M, Ganesan G, Toh D, Chan CL. Usage Pattern of Carbamazepine and Associated Severe Cutaneous Adverse Reactions in Singapore Following Implementation of HLA-B*15:02 Genotyping as Standard-of-Care. Front Pharmacol. 2020 May 7;11:527. doi:10.3389/fphar.2020.00527.PMID: 32457602; PMCID: PMC7221117.
Ramasamy SN, Korb-Wells CS, Kannangara DR, Smith MW, Wang N, Roberts DM, Graham GG, Williams KM, Day RO. Allopurinol hypersensitivity: a systematic review of all published cases, 1950-2012. Drug Saf. 2013 Oct;36(10):953-80. doi: 10.1007/s40264-013-0084-0.
Saito Y, Stamp LK, Caudle KE, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for human leukocyte antigen B (HLA-B) genotype and allopurinol dosing: 2015 update. Clin Pharmacol Ther. 2016 Jan;99(1):36-7. doi: 10.1002/cpt.161. Epub 2015 Jul 16.
Role of HLA-B*5801 genotyping prior to initiation of allopurinol. Singapore: Health Sciences Authority; 2016 [updated: 23 Mar 2016; accessed: 15 Sept 2024]. Available from: https://www.hsa.gov.sg/announcements/dear-healthcare-professional-letter/roleof-hla-b-5801-genotyping-prior-to-initiation-of-allopurinol
Allopurinol-induced severe cutaneous adverse reactions and the role of HLA-B*5801 genotyping – a reminder. Singapore: Health Sciences Authority; 2021 [updated: 21 Dec 2021; accessed: 18 Oct 2023]. Available from: https://www.hsa.gov.sg/announcements/safety-alert/allopurinol-induced-severe-cutaneous-adversereactions-and-the-role-of-hla-b-5801-genotyping-a-reminder
ACE Clinical Guidance. Gout – achieving the management goals. [updated: 14 December 2023; accessed: 15 Sept 2024]. Available from gout-achieving-the-management-goal-dec2023.pdf (ace-hta.gov.sg)
Lee CR, Luzum JA, Sangkuhl K, Gammal RS, et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin Pharmacol Ther. 2022 Nov;112(5):959-967. doi: 10.1002/cpt.2526. Epub 2022 Feb 8.
Pereira NL, Cresci S, Angiolillo DJ, et al. American Heart Association Professional/Public Education and Publications Committee of the Council on Genomic and Precision Medicine; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Peripheral Vascular Disease; and Stroke Council. CYP2C19 Genetic Testing for Oral P2Y12 Inhibitor Therapy: A Scientific Statement From the American Heart Association. Circulation. 2024 Aug 6;150(6):e129-e150. doi: 10.1161/CIR.0000000000001257. Epub 2024 Jun 20.
Wang Y, Meng X, Wang A, et al. CHANCE-2 Investigators. Ticagrelor versus Clopidogrel in CYP2C19 Loss-of-Function Carriers with Stroke or TIA. N Engl J Med. 2021 Dec 30;385(27):2520-2530. doi: 10.1056/NEJMoa2111749. Epub 2021 Oct 28.
Narasimhalu K, Ang YK, Tan DSY, et al. Cost Effectiveness of Genotype-Guided Antiplatelet Therapy in Asian Ischemic Stroke Patients: Ticagrelor as an Alternative to Clopidogrel in Patients with CYP2C19 Loss of Function Mutations. Clin Drug Investig. 2020 Nov;40(11):1063-1070. doi: 10.1007/s40261-020-00970-y.
Wong, E., Bertin, N., Hebrard, M. et al. The Singapore National Precision Medicine Strategy. Nat Genet 55, 178–186 (2023). https://doi.org/10.1038/s41588-022-01274-x
Moratorium on Genetic Testing and Insurance. Singapore: Ministry of Health; 2021 [updated: 6 Jun 2022; accessed: 15 Sept 2024]. Available from: https://www.moh.gov.sg/resources-statistics/moratorium-on-genetic-testing-and-insurance
Regulations, Guidelines and Circulars. Singapore: Ministry of Health; 2021 [updated: 7 Nov 2022; accessed: 15 Sept 2024]. Available from: https://www.moh.gov.sg/licensing-and-regulation/regulations-guidelines-and-circulars/details/updates-to-code-ofpractice-on-the-standards-for-the-provision-of-clinical-geneticgenomic-testing-services-and-clinical-laboratory-genetic-genomic-testing-services
HM Chen, EAG Lo. Pharmacogenomics in a Nutshell for General Practitioners. Singapore Family Physician 2024; 50(1):41-47.
Special thanks to Dr Kaavya Narasimhalu (Consultant, National Neuroscience Institute) for her valuable comments for this article and her camaraderie in the pharmacogenomics implementation journey.
Dr Wong Pei Shieen is a clinical pharmacist at Singapore General Hospital. She has a keen interest in optimising pharmacotherapy using precision therapeutics. She has completed the ASHP Pharmacogenomics Certification and is the Pharmacy Lead for the SingHealth Pharmacogenomics Implementation initiative. GPs who would like more information on pharmacogenomics can contact Dr Wong at [email protected].
Associate Professor Lee Haur Yueh is a Senior Consultant at the Department of Dermatology, Singapore General Hospital as well as the Director of the Allergy Centre. His clinical interests are in the prevention and management of severe drug reactions and allergies.
GPs can call the SingHealth Duke-NUS Genomic Medicine Centre for appointments at the following hotlines, visit the webpage for more information.
Singapore General Hospital: 6326 6060
KK Women's and Children's Hospital: 6692 2984
National Cancer Centre Singapore: 6324 8798
National Heart Centre Singapore: 6704 2222
