Paediatric brain tumour care is mandatory for children. It starts with early detection, implementation of new tumour molecular diagnostics and therapies and post treatment survivorship clinics according to KK Women's and Children's Hospital.
Brain tumours are the commonest solid tumours in children and are the leading cause of cancer death inchildhood. According to data from the Singapore Cancer Registry, from 2008 to 2012, central nervous systems (CNS) tumours were the second most frequently occurring cancer in children (18.3%) after leukaemias (35.3%).1
While advances in neurosurgery, radiotherapy and chemotherapy have raised survival rates of children with brain tumours, mortality and morbidity nonetheless remain significant. There is still much work to be done to improve cure rates and minimise treatment-related side effects wherever possible.
FACILITATING EARLY RECOGNITION BY PRIMARY HEALTHCARE PROVIDERS `
The first step in advancing care for paediatric brain tumours is to facilitate early recognition by primary healthcare providers. Studies have shown that brain tumours in childhood have the greatest delay in diagnosis2,3, likely due to the myriad of symptoms they could present with3,4, including more subtle symptoms of gradually increasing intracranial pressure or developmental regression, or more glaring symptoms of cranial nerve palsies or seizures.
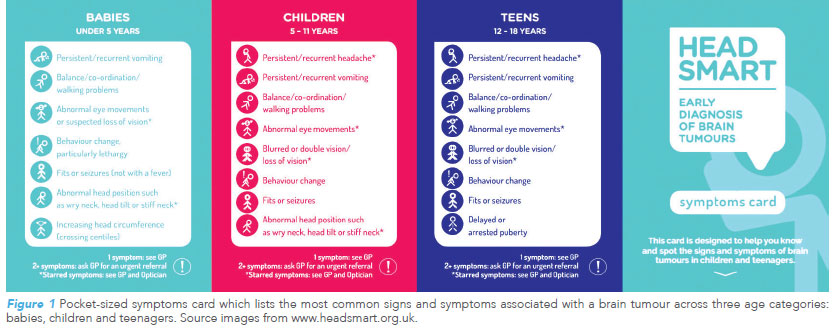
Globally, countries are recognising the value of facilitating early recognition. The “HeadSmart: Be Brain Tumour Aware” campaign was launched in June 2011 across the United Kingdom as a quality improvement strategy, to raise awareness among the public and healthcare professionals to recognise the signs and symptoms which should trigger an early referral for evaluation and neuroimaging.
In combination with revised National Health Service referral guidelines published in 2008, the campaign was successful in reducing the total diagnostic interval from a pre-campaign median of 14 weeks to 6.7 weeks5. Centres in the United States of America are also intending to adapt the same programme to reduce the time interval between symptom onset and diagnosis for their patients2.
NEW APPROACHES TO CLASSIFICATION AND TREATMENT OF PAEDIATRIC BRAIN TUMOURS
Paediatric brain tumours make up a heterogeneous basket of tumours – from slow-growing, low-grade gliomas to malignant medulloblastomas. The more common brain tumours in childhood include:
- Medulloblastoma
- Low-grade gliomas
- High-grade gliomas and diffuse midline gliomas
- Germ cell tumours
- Ependymoma
- Craniopharyngioma
- Less commonly, other embryonal tumours such as atypical teratoid rhabdoid tumours (ATRT) and embryonal tumours with multi-layered rosettes (ETMR)
Refining the classification of brain tumours
Traditionally, it was thought that the aggressiveness of the disease is dictated by tumour histology and grade, and the extent of the tumour or the presence of metastasis.
However, it is now recognised that tumour molecular information predicts tumour behaviour more accurately. This is made possible due to advances in the last decade in
molecular diagnostics (genomic, epigenetic and transcriptomic profiling) and increased global collaborations and knowledge sharing.
These advances have revolutionised the genomic landscape for paediatric brain tumours6,7 and driven important revisions in the World Health Organization (WHO) 2016 classification of CNS tumours8. The revisions introduced integrated diagnoses incorporating both conventional histology and newer genomic features, and examples of the classifications include “medulloblastoma, WNT-activated”, “ependymoma, RELA fusion-positive” and “diffuse midline glioma,
H3K27M mutant”.
APPLICATION OF MOLECULAR DIAGNOSTICS
Molecular Subgrouping
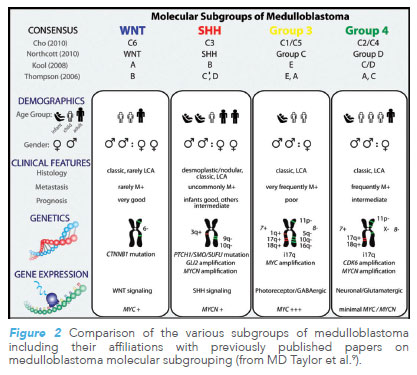
Clinical application of molecular data can be seen in the molecular subgrouping of paediatric brain tumours. A 2012 landmark paper9 for molecular subgrouping in medulloblastoma, the commonest malignant brain tumour of childhood, established that tumour behaviour was better predicted through molecular subgroups instead of conventional histological subgroups.
Among the four molecular subgroups, the WNT subgroup had the best prognosis, whereas outcomes for Group 3 medulloblastoma were the worst.
This molecular classification is now widely accepted and assimilated into current literature and clinical trials. Since then, molecular subgrouping has also been used for other brain tumour groups such as ependymoma10, ATRT11 and diffuse intrinsic pontine glioma (which is now incorporated into the entity of diffuse midline gliomas)12.
Improved prognostication allows more accurate riskstratification and, consequently, better treatment stratification. In favourable-risk subgroups, the goal is to reduce treatment morbidity in patients whom we foresee to be long-term survivors by introducing treatment reduction; conversely, in poor-risk subgroups, the goal is to improve cure rates by exploring novel treatment regimens.
Novel Therapeutics
Identification of signature tumour mutations has enabled targeted therapy to be employed for patients who previously had little or no further treatment options6. For example, BRAF gene alterations in paediatric gliomas, such as BRAF V600E mutations or KIAA-1549 BRAF fusion, provide a target for BRAF inhibitors and/or MEK inhibitors.
Early phase trials in paediatric gliomas have shown optimistic tumour responses and manageable drug toxicity13,14. These drugs are now a promising treatment option for patients whose gliomas bear these BRAF alterations and who have failed conventional therapy7,15.
ADOPTING A MULTIDISCIPLINARY APPROACH TO PAEDIATRIC BRAIN TUMOUR CARE
Beyond advances in tumour diagnostics and treatment, adopting a multidisciplinary approach towards paediatric brain tumour care is important. From diagnosis to treatment and supportive care, and to post-treatment surveillance, KKH has a comprehensive team coordinating and delivering care for paediatric patients with brain tumours.
Tumour board meeting
A multidisciplinary tumour board meeting serves as a platform for the team of radiologists, pathologists, oncologists, neurosurgeons and radiation oncologists to jointly review neuroimaging, pathology slides, and discuss the appropriate treatment – surgery, radiotherapy, and/or chemotherapy – as the optimal treatment may differ from case to case, depending on tumour histology, tumour location, disease extent and the age of the child.
The ancillary team
For a child who is embarking on chemotherapy, an ancillary healthcare team comprising oncology pharmacists, dietitians and oncology nurses provides counselling and care during the child’s treatment. In addition, a paediatric brain tumour resource nurse coordinates complex care with multiple treatment visits and investigations, and offers frontline advice and emotional support to patients and their families.
Psychosocial support and neurorehabilitation
 The psychosocial needs of a child and his/her family who have received the life-changing news of a brain tumour diagnosis cannot be over-emphasised. At KKH, medical social workers and case workers from the Children’s Cancer Foundation (CCF) jointly support the child, parents and siblings throughout their journey from diagnosis. The CCF Resource Centre and teens’ room are also available to patients who are receiving treatment in the oncology wards and Day Therapy Centre, and often become the highlight of their treatment visits.
The psychosocial needs of a child and his/her family who have received the life-changing news of a brain tumour diagnosis cannot be over-emphasised. At KKH, medical social workers and case workers from the Children’s Cancer Foundation (CCF) jointly support the child, parents and siblings throughout their journey from diagnosis. The CCF Resource Centre and teens’ room are also available to patients who are receiving treatment in the oncology wards and Day Therapy Centre, and often become the highlight of their treatment visits.
In 2017, the CCF Psychosocial and Supportive Care Programme was also established at KKH, where the Nutrition and Dietetics Department, Rehabilitation Department, and Psychology Service have been providing holistic and supportive care for paediatric oncology patients. This programme is especially relevant to brain tumour patients, who may experience neurological deficits and cognitive difficulties due to the tumour or raised intracranial pressure, from a tumour surgery or as a long-term side effect from radiotherapy and/or chemotherapy. Knowing the plasticity of the paediatric brain, well-organised neurorehabilitation can greatly improve the functional status of a child with neurological deficits or help to prevent further deterioration of functional disabilities.
In KKH, neurorehabilitation is carried out by paediatric therapists through physiotherapy, occupational therapy, and speech therapy, in tandem with assessments by a neurorehabilitation physician, and may continue even after the child has completed the cancer treatment and is preparing to re-assimilate into school. Neuropsychology assessments are also made at regular intervals to monitor for neurocognitive deficits such that early interventions for learning difficulties can be recommended at appropriate timings.
Post-treatment surveillance
As cure rates for paediatric neuro-oncology improve, the burden ripple effects of cure become more apparent when long-term side effects develop from treatment. These may include neurocognitive deficits, endocrinopathies from cranial irradiation, hearing loss, subfertility from chemotherapy and more. The KKH Survivorship Clinic and Late Effects Clinic incorporate comprehensive post-treatment surveillance for an early detection of these longterm complications, as well as provide patient education and relevant referrals to subspecialties to manage these complications.
CONCLUSION Advancement of paediatric brain tumour care requires a team effort from different aspects of the healthcare system. This starts with early detection by primary care professionals, implementation of new tumour molecular diagnostics and therapies, application of multidisciplinary approaches towards management of the condition in tertiary centres, and post-treatment surveillance in survivorship clinics. |
REFERENCES
1. Trends in Cancer Incidence in Singapore 2008-2012, Singapore Cancer Registry Report No. 8.
2. Coven SL et al. Delays in diagnosis for children with newly diagnosed CNS tumors. Neurooncol Pract. 2018 Nov;5(4): 227-233.
3. Goldman RD et al. Improving diagnosis of pediatric CNS tumours: aiming for early detection. CMAJ. 2017 Mar 27;189(12):E459-E463.
4. Wilne S et al. Presentation of childhood CNS tumours: a systematic review and meta-analysis. Lancet Oncology 2007; 8:685-95
5. Walker D et al. A new clinical guideline from the RCPCH with a national awareness campaign accelerates brain tumor diagnosis in UK children- -”HeadSmart: Be Brain Tumour Aware”. Neuro Oncol. 2016 Mar;18(3):445-54.
6. Gajjar A et al. Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape. J Clin Oncol. 2015 Sep 20;33(27):2986-98.
7. Pollack I et al. Childhood brain tumors: current management, biological insights, and future directions. J Neurosurg Pediatr 2019; 23: 261-273.
8. Louis DN et al. The 2016 WHO Classification of Tumors of the CNS: a summary. Acta Neuropathol (2016) 131: 803-820.
9. MD Taylor et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012 Apr; 123(4): 465–472.
10. Pajtler KW et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell. 2015 May 11;27(5):728-43.
11. Johann PD, et al. ATRTs Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes. Cancer Cell. 2016 Mar 14;29(3):379-393.
12. Buczkowicz et al. Genomic analysis of DIPGs identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014 May;46(5):451-6.
13. Kieran MW et al. Phase 1 study of dabrafenib in pediatric patients with relapsed or refractory BRAF V600E HGG, LGG, LCH, and other solid tumors. ASCO Annual Meeting. Chicago, IL, USA: 29 May–2 June 2015.
14. Hargrave D et al. Dabrafenib in pediatric patients with BRAF V600–positive HGG. Journal of Clinical Oncology 2018 36:15_suppl, 10505-10505
15. Sturm D et al. Pediatric Gliomas: Current Concepts on Diagnosis, Biology, and Clinical Management. J Clin Oncol. 2017 Jul 20;35(21):2370-2377.
16. Low SYY et al. DTI fusion with conventional MR imaging in intra-operative MRI suite for paediatric brainstem glioma biopsy. Childs Nerv Syst. 2018 Jan;34(1):19-21.
17. Pfaff E et al. Brainstem biopsy in pediatric DIPG in the era of precision medicine: the INFORM study experience. Eur J Cancer. 2019 Jun;114:27-35.
By: Dr Enrica Tan is a Consultant in the Haematology/Oncology Service at KK Women’s and Children’s Hospital (KKH). She completed her fellowship in paediatric neurooncology in Great Ormond St Hospital, London, and is currently one of the leads of the paediatric neuro-oncology multidisciplinary care in KKH. Dr Tan is also the Vice Chair of the Brain Tumour Study Group of Paediatric Oncology Group (Singapore) and serves as one of the medical advisors in the Brain Tumour Society (Singapore).
GPs can call for appointments through the GP Referral Hotline at 6692 2984 for more information about the department.
