CAR T-cell therapy is a novel approach in the treatment of haematological malignancy. Find out more about this treatment and the care pathways surrounding its delivery, as shared by the SingHealth Duke-NUS Cell Therapy Centre.
With rising cancer incidence and improvements in patient survival outcomes through novel therapies such as cellular immunotherapy, general practitioners are poised to take on an increasing role in partnering haematologists or oncologists in joint cancer survivorship programmes. Find out more about CAR T-cell therapy as a novel approach in the treatment of haematological malignancy and the care pathways surrounding its delivery.
INTRODUCTION
The evolving field of cellular immunotherapy is revolutionising the cancer treatment landscape.
Chimeric antigen receptor (CAR) T-cell therapy is a form of adoptive T-cell therapy that has been recently introduced in the treatment armamentarium of haematological malignancies. It involves ex vivo engineering of the patient's autologous T cells to equip them with receptors targeting specific antigens on cancer cells, and subsequently infusing these genetically modified T cells back into patients to bring about cancer-directed cytotoxicity.
Here we will focus on the patient journey, using CAR T-cell therapy as an example of cellular immunotherapy for diffuse large B-cell lymphoma (DLBCL), and elaborate on the role of general practitioners (GPs) as cell therapies become more common in clinical practice.
PREVALENCE OF DLBCL IN SINGAPORE
In Singapore, tisagenlecleucel (Kymriah), an anti- CD19 CAR T-cell therapy, is currently approved and available for use in young adults (< 25 years) with relapsed/refractory (R/R) acute B-cell lymphoblastic leukaemia (B-ALL) and adults with DLBCL.
DLBCL is the most common form of aggressive lymphoma in Singapore. The median age of DLBCL patients locally is 60-65 years. According to the World Health Organization's guidance on classifying cancers, DLBCL accounts for 30-40% of newly diagnosed cases of non-Hodgkin lymphoma globally.
Early symptoms of DLBCL
It is paramount that patients who are suspected to have DLBCL are referred immediately. Early symptoms of DLBCL include lumps in the neck, armpits or groin. Some patients may not show any obvious symptoms except fevers that may persist and unexplained weight loss.
WHO CAN BENEFIT FROM CAR T-CELL THERAPY?
Patients diagnosed with DLBCL will undergo standard of care therapy with an anti-CD 20 monoclonal antibody and chemotherapy. The majority will respond and go into remission. However, 30-40% will relapse and among those who do, up to 50% may still be refractory to salvage therapies with dismal outcomes.
CAR T-cell therapy offers an alternative form of treatment for such patients with a complete remission rate of 40%.
However, the process of delivering and administering CAR T-cell therapy to a patient is complex, as illustrated in Figure 1.
Even if patients meet the criteria for CAR T-cell therapy, they still need to have a consultation with a haematologist to ensure that they would benefit from this therapy, and are willing to accept the toxicities associated with it.
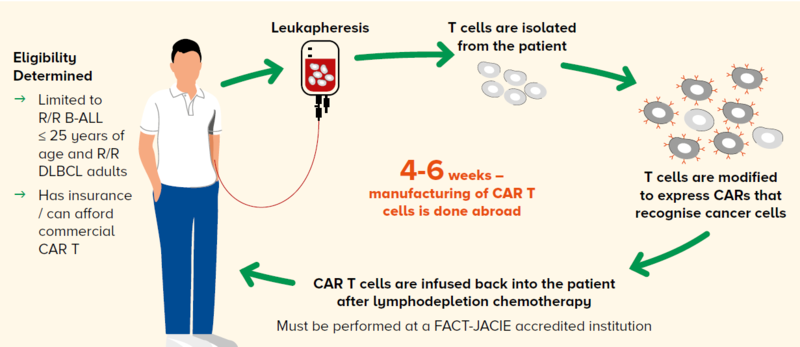
Figure 1 The CAR T workflow at the Singapore General Hospital Department of Haematology
CELLULAR IMMUNOTHERAPY AT THE SINGAPORE GENERAL HOSPITAL DEPARTMENT OF HAEMATOLOGYAt Singapore General Hospital (SGH), the Department of Haematology is accredited to administer cell therapy products to patients. The hospital is equipped with the appropriate infrastructure, as well as a multidisciplinary team comprising trained individuals, to care for these patients. Currently, every patient who might benefit from CAR T-cell therapy is presented at our Haematology Lymphoma Tumour Board and subsequently at our Haematopoietic Cell Therapy and Transplant Programme (HCTTP) meetings. We have treated ten patients with CAR-T cell therapy since last year at SGH. |
THE CELLULAR IMMUNOTHERAPY PATIENT JOURNEY
Counselling on the treatment plan
The patient will need to be counselled on the various steps:
The process of leukapheresis to obtain the T cells from the patient for manufacturing of CAR T cells
The possibility of receiving bridging therapy while waiting for the CAR T cells to arrive – this could comprise more immunochemotherapy, radiotherapy or a combination
The admission for lymphodepletion chemotherapy before the infusion of CAR T-cell therapy and the toxicities
The discharge planning and follow-up
Managing the patient's emotions
The emotions that the patient experiences during this whole process cannot be overemphasised. There is much anxiety and expectation, and it is paramount that the clinician spends time explaining this complex process to them.
It is challenging for the patient to comprehend how these CAR T cells are manufactured with a turnaround time of six weeks, and to accept the unique toxicities associated with CAR T-cell therapy.
Understanding and managing acute toxicities
These therapies are associated with unique acute toxicities of cytokine release syndrome (CRS) and neurological toxicity, also referred to as CAR-related encephalopathy syndrome (CRES) or immune effector cell-associated neurotoxicity syndrome (ICANS), that are not typically seen with traditional anticancer therapies.
Cytokine release syndrome (CRS)
CRS is the most common acute adverse event associated with CAR T-cell therapy.
CRS is a systemic inflammatory response triggered by the release of cytokines by CAR T cells following their activation upon tumour recognition in vivo. The CAR T cells also activate bystander immune cells such as macrophages, which in turn release inflammatory cytokines and contribute to the pathophysiology of CRS.
Presentation
CRS typically manifests with constitutional symptoms of fever, myalgias, rigours, fatigue and loss of appetite, but can lead to multiorgan dysfunction in more severe cases.
Management
CRS is completely reversible if managed appropriately. Serum IL-6 levels have been shown to correlate with the severity of CRS, and the blockade of IL-6 with tocilizumab, an anti-IL-6 receptor antibody, can reverse CRS.
Immune effector cell-associated neurotoxicity syndrome (ICANS)
ICANS is less well-understood than CRS. Like CRS, cytokines, chemokines, and the degree of CAR T cell expansion have been associated with more severe neurotoxicity.
Presentation
It typically presents as a toxic encephalopathy with word-finding difficulty, aphasia and confusion but can progress in more severe cases to depressed levels of consciousness, comas, seizures, motor weakness and cerebral oedema.
Management
Because of the limited understanding of the pathophysiology, ICANS is primarily managed with supportive care for low-grade toxicity, and corticosteroids are frequently used for more severe grades. Like CRS, ICANS is also completely reversible in most patients and tends to have a self-limited course.
Other toxicities
In addition, delayed toxicities such as prolonged cytopenias, risk of opportunistic infections, and on-target but off-tumour effects such as B-cell aplasia are observed with anti-CD19 CAR T products.
Not all cell and gene therapies have gained FDA approval. Many are still in the pre-clinical stage of development and are required to undergo the rigours of clinical trial before they are truly proven to be safe and efficacious. However, with such limelight on cell therapy, patients are always attracted to new therapies and curious to understand if they are available in Singapore. Unfortunately, this is often the response of desperate patients – those who have failed line after line of therapies – and they often fall prey to these unproven cell therapies. Educating and referring patients As a GP, you may encounter circumstances where your opinion is sought regarding cell therapies. Your role will thus be paramount in educating the patient and their relatives that unproven cell therapies may not just be costly, but can also have severe devastating effects on their health even resulting in death. You may also play a critical role in directing the patient to specialist centres in the event of any ambiguity. Working with specialists for shared care Integrating care The patient journey may also be emotionally challenging and physically demanding. Here, there can be greater integration of care between the GP and specialist in the many pre- and post-treatment visits as illustrated in Figure 2. Establishing channels of communication The channel of communication between GPs and specialists should be well established such that the patient is not left to communicate with both parties independently. Patients should be assured that the GP is aware of what is happening at the hospital, and is confident in providing the extra support in the community and advising the patient to go to the hospital when appropriate. |
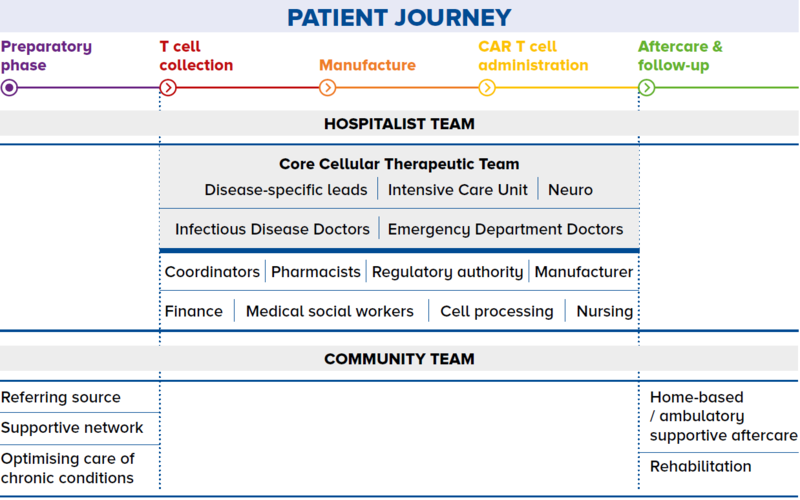
Figure 2 The patient journey through a shared care programme
THE FUTURE OF CELL THERAPIES
Current FDA-approved therapies
There are currently five FDA-approved second generation CAR T-cell therapies as shown in Table 1.
The current drug labels are for B-ALL, B-lymphoproliferative disorders and multiple myeloma. Current approved CAR T-cell therapies are autologous in nature and for use in the R/R setting.
Many other CAR T-cell therapies are being investigated in ongoing clinical trials, and we anticipate others to be approved in future for other haematological cancers and solid tumours.
'Off-the-shelf' CAR T-cell therapy products
There are also ongoing clinical trials looking at 'off-the-shelf' CAR T-cell therapy products – where instead of using autologous T cells for the manufacturing of these products, researchers and clinicians are using other immune cells from healthy donors such as natural killer cells (NK cells) and gamma delta T cells (GDT cells).
The rationale for doing so is to make this form of therapy more accessible for all patients with a faster turnaround time, and hopefully be able to provide starting cells that are 'healthier' as they are not from the patient.
There is also much research activity on how the CARs can be further modified to make them more efficient in killing cancer cells and to make them safer with less risk of CRS and ICANS.
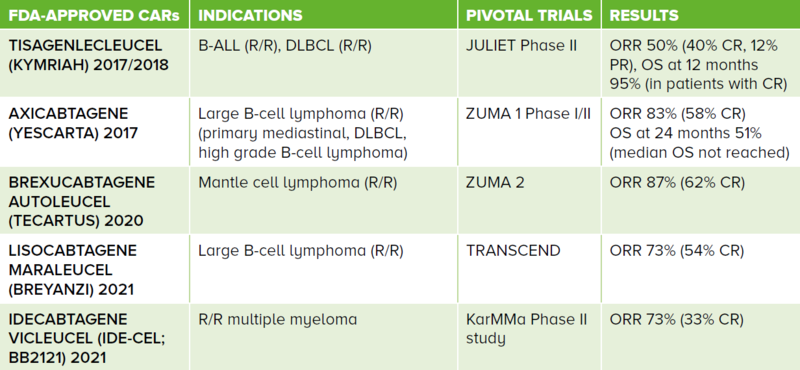
Table 1 FDA-approved second generation CARs and their respective indications. These are all autologous-derived cell therapy products. The first four CARs target the CD19 antigen while the fifth targets the B-cell maturation antigen.
ALL: acute lymphoblastic leukaemia, DLBCL: diffuse large B-cell lymphoma, R/R: relapsed/refractory, ORR: overall response rate, CR: complete response, PR: partial response, OS: overall survival
CONCLUSION
It is evident that immune cell therapy is here to stay and the potential to use these therapies for other diseases is promising. In Singapore, this is a new paradigm in medicine and it is important for all GPs to be aware of this shift – where we are no longer just using pills to treat patients, but also using living cells to cure them of diseases.
Assistant Professor Francesca Lim is a Consultant Haematologist at Singapore General Hospital (SGH), Deputy Head and Principal Lead (Education) at the SingHealth Duke-NUS Cell Therapy Centre and the Assistant Medical Director at the Cell Therapy Facility, Health Sciences Authority. She spent two years training at the MD Anderson Cancer Center in CAR T-cell therapy, particularly in CAR-NK cell therapy. She has been instrumental in supporting and coordinating the Cell Therapy Programme at the SGH Department of Haematology, both at the clinical and research levels.
Dr Chen Yunxin is a Consultant Haematologist at Singapore General Hospital, and Co-Lead (Education) and Clinical Deputy Lead (Adult Blood Cancer) at the SingHealth Duke-NUS Cell Therapy Centre. She specialises in the care of patients with multiple myeloma and lymphoproliferative disorders. She completed her Health Manpower Development Plan on immunotherapy at the Memorial Sloan Kettering Cancer Center where she was involved in developing novel cellular therapeutics for treatment of multiple myeloma as well as myeloma CAR T cell clinical trials. She works with a dedicated team of physicians and allied health professionals in the Haematopoietic Stem Cell Transplant Programme to deliver CAR T cells to patients as well as to develop outpatient transplant services.
GPs can contact the SingHealth Duke-NUS Cell Therapy Centre at [email protected] to know more about the available cell therapies and clinical trials on the SingHealth campus.
