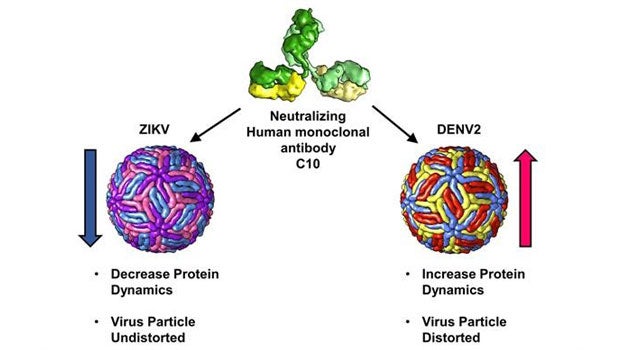
Structural analysis of the antibody C10 reveals distinctly different mechanisms of action on the Zika and Dengue (DENV2) virus.
NUS scientists have discovered that a single antibody can neutralise different flaviviruses in multiple ways including a novel mode of neutralisation involving distortion of the viral surface.
Flavivirus infections are the most prevalent arthropod-borne diseases and are a major global public health burden. Dengue (DENV) and Zika (ZIKV) are two viruses belonging to the Flaviviridae family. Patients that have recovered from dengue infections develop neutralising antibodies against DENV viruses. Recently, an important class of antibodies, that were isolated from dengue-recovered patients, were found to also possess the capability to neutralise Zika virus with differing efficacy.
A research team led by Prof Lok Shee-Mei from the Department of Biological Sciences and the Duke-NUS Medical School, National University of Singapore, has shown that the same antibody can neutralise both the Zika and the Dengue viruses in two distinct ways (see Figure). This work involved a collaboration with Prof Ganesh Srinivasan ANAND who is now with the Pennsylvania State University. The antibody neutralised the Zika virus by locking the virus and restricting its protein motion. In contrast, the same antibody neutralised the Dengue virus serotype 2 (DENV2) by distorting and enhancing its protein motion. The researchers used a combination of cryo-electron microscopy (cryo-EM) and hydrogen/deuterium exchange mass spectrometry (HDXMS) to visualise and capture these morphological changes when the Zika and the DENV2 virus particles were bound by the antibody. Moreover, the team found that the antibody exhibit preference for binding to its epitope sites on the DENV2 surfaces.
There are 180 copies of envelope proteins on mature Dengue and Zika virus particles and they are arranged in a unique pattern that give rise to icosahedral symmetry. Antibody recognition sites are made up of specific small parts on the surface of an envelope protein. This means that a virus particle possesses many antibody recognition sites, known as epitopes. An antibody can neutralise a virus through a combination of mechanisms such as inhibition of attachment (binding to virus surface preventing the virus from infecting a cell) or inhibition of fusion (binding and blocking the structural changes required for infecting a cell). In this work, the research team discovered a new virus neutralisation mechanism, known as virus particle distortion.
Dr Lim Xin Xiang, a member of the research team and the first author of the paper said, “Proteins and other biomolecules in the virus particle are not static and exist in a state of continuous motion known as protein dynamics. By examining how the human antibody C10, at different concentrations, interacts with the DENV2, we found that dengue presents a dynamic and nonuniform epitope landscape for C10 binding.”
Dr Shu Bo, another member of the research team said, “It is a surprising finding for us that an antibody could enhance the dynamic motion on the viral surface so badly that it gets distorted, potentially neutralising it.”
The ability of an antibody to induce large-scale viral structural changes has not been studied widely and the team seeks to further investigate the impact of virus particle distortion on efficiency of antibody-mediated virus neutralisation.
Prof Lok said, “It is delightful to collaborate with Ganesh Anand’s lab. We will continue to use integrated bio-physical methods to study how antibodies can kill viruses. The more we learn, the more we are aware that we have only touched the tip of the iceberg.”
Reference: X-X Lim; B Shu; SJ Zhang; AWK Tan; T-S Ng; X-N Lim; VS-Y Chew; J Shi; G Screaton; S-M Lok*; GS Anand*, “Human antibody C10 neutralizes by diminishing Zika but enhancing dengue virus dynamics” CELL Volume 184 Pages 1-14 DOI:10.1016/j.cell.2021.11.009 Published: 2021.












