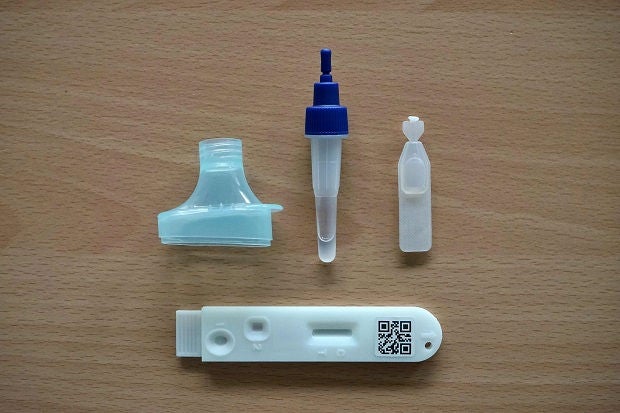
This saliva antigen COVID-19 test will be an even more convenient option compared to nasal swabs.
A saliva-based COVID-19
antigen rapid test (ART)
technology co-developed by
the SingHealth Duke-NUS
Academic Medical Centre
and the National University
of Singapore (NUS) will offer a more
pleasant alternative to nasal swabs.
Officially known as PASPORT (Parallel Amplified Saliva rapid POint-of-caRe Test), this easy-to-use and painless test is highly accurate. Compared to the gold standard Polymerase Chain Reaction (PCR) test, PASPORT’s sensitivity in detecting the SARS-CoV-2 virus is 97 per cent, and its specificity (to detect a true negative result) is 90.6 per cent. These results are comparable to ART nasal swabs available in Singapore.
While PASPORT is not the first test that can be used for saliva testing, it vastly outperforms the handful of existing options, which have not been accurate enough to be rolled out at large scale.

One of the major limitations of existing saliva tests is that they are accurate only after prolonged fasting, such as in the morning after waking up. “Should participants eat, drink or brush their teeth, the concentration of viral particles in the saliva drops too drastically for an accurate result. A comparison against two other existing test kits showed that sensitivity dropped to approximately 60 per cent and 20 per cent respectively after food,” said Dr Danny Tng (pictured), Medical Officer, Department of Infectious Diseases, Singapore General Hospital (SGH). He is also an adjunct Research Fellow at Duke-NUS’s Emerging Infectious Diseases (EID) Programme and one of the key inventors of PASPORT.
The other inventors include Associate Professor Melvin Chua from National Cancer Centre Singapore (NCCS) and Duke-NUS Medical School, Professor Jenny Low from SGH and Duke-NUS Medical School, Professor Ooi Eng Eong from Duke-NUS Medical School, Professor Soo Khee Chee from NCCS and Duke-NUS Medical School, and Professor Zhang Yong from NUS.
PASPORT can be used at any time of the day, even after meals. Like other ARTs, it uses nanoparticles to bind the virus. In addition, it adds a second type of nanoparticle that binds the first set of nanoparticles to amplify the signal, allowing it to be more sensitive than other lateral flow tests. Using its unique viral capture system with ACE2 proteins, it is also able to pick up COVID-19 variants, including Delta.
This new kit looks similar to a regular ART kit. The main difference is that instead of a nasal swab, users will collect a small amount of saliva in a funnel, drop some saliva in the first channel of the test kit, and wait for up to 15 minutes for results. If results are negative, they will drop more saliva into the second channel of the test kit and wait for another 15 minutes for amplified results. Therefore, this self-administered test can be completed in as soon as 15 to 30 minutes.








Dr Tng noted that globally, there is a high demand for ART over PCR testing. This is because the latter requires specially trained personnel for sample collection, processing and interpretation of results, is costlier ($150 per test on average), and takes up to a day to get results.
“With new COVID-19 anti-viral oral medications like Molnupiravir, it is very important to administer these drugs within the first few days of illness to achieve the best outcomes. As PCR takes a longer time, you could miss a critical window for treatment,” he explained.
Furthermore, saliva antigen tests reduce the margin of error that occurs when the nasal swab is not done properly — for instance, when it is not be inserted deep enough into the nasal cavity. “Some studies have shown a 10 per cent difference in accuracy when a professional does the nasal swab compared to when users do it on their own,” Dr Tng said.
The saliva test may also be easier to administer on children who may find nasal swabs uncomfortable. This convenient testing method is also important since there is currently no vaccination available for very young children.
“A test like PASPORT that can be selfadministered or used on-site at the primary care setting may mitigate the need for cases to be managed at the hospitals,” said Prof Ooi.
Manufactured by Singapore-based company Digital Life Line Pte Ltd, PASPORT is expected to be available for consumer use by the end of 2022.
Get the latest updates about Singapore Health in your mailbox! Click here to subscribe.













 Get it on Google Play
Get it on Google Play