HealthXchange will NEVER ask you to transfer money over a call. If in doubt, call the 24/7 ScamShield helpline at 1799, or visit the ScamShield website at www.scamshield.gov.sg.
A new cancer drug has been approved for use offering hope to some 120 lung cancer patients diagnosed here every year who do not respond to current treatments.
Ceritinib, which comes in the form of a pill, targets a particular gene mutation responsible for the disease in around 8 per cent of lung cancer patients in Singapore.
Clinical trials have been conducted at 20 centres across nine countries, including the National Cancer Centre Singapore (NCCS), and the Health Sciences Authority has approved it for treatment here. Over the past four years, early-phase trials here involved 36 patients resistant to or intolerant of Crizotinib, the only other drug that works against the same mutation.
For such patients with Stage 4 lung cancer, their median survival rate was just over four years in total - or 49.4 months. They also achieved disease control for a median of 17.4 months - during which there was no growth of the cancer.
With Crizotinib alone, the average time the disease could be controlled ranged from six to 11 months. Most patients with late-stage lung cancer do not survive longer than a year, when treated only with chemotherapy.
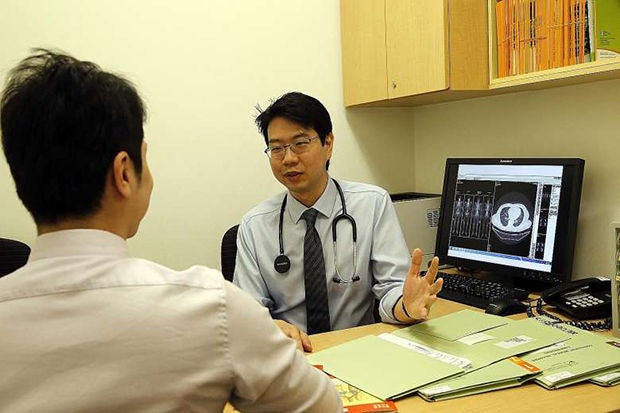
"It's great we've another option that is now available for patients who are failing the first-line treatment," said Dr Daniel Tan, consultant at NCCS' division of medical oncology and one of the lead investigators of clinical trials done here.
Mr Samsuri Abdul Haris, 40, a barber, said his father - who has been part of the drug trial for the last two years - has benefited. His 70-year-old father was diagnosed with Stage 4 lung cancer in 2013.
"The doctor has showed me the tumour has become smaller, which is very good news. (My dad) has already put on weight," he said.
Get the Health Buddy App
© 2025 SingHealth Group. All Rights Reserved.














 Get it on Google Play
Get it on Google Play