are an excellent option for patients with an advanced heart failure.
As a result of an ageing population and advances in the treatment of heart diseases, an increasing number of patients are reaching the AHA/ACC advanced stage D, also known as the end-stage, refractory or the stage of terminal heart failure.
These patients are often characterised by a poor quality of life, dyspnoea at rest despite optimal medical therapy, an objective evidence of structural heart disease, multiple hospital admissions and early signs of deteriorating function in the kidneys and liver. They have a prognosis that is worse than many cancers, with very few surviving beyond a period of 2 years.
There are only a few available options. Heart transplantation provides excellent results, but the supply of donor organs is very limited in Singapore. Only younger patients, aged 60- years-old and below, are eligible with a few exceptions, and the patients used to pass away while waiting.
In 2008, a new option was made available. The Food and Drug Administration (FDA) approved the use of a new generation continuous flow Left Ventricular Assist Device (LVAD), which doubled the survival rate as compared to the older generation of LVADs, and it grew rapidly all over the world.
By the end of 2014, more than 20,000 Heartmate IIs had already been implanted worldwide. It has become the standard of care in advanced countries and is still continuing to grow.
In 2009, the National Heart Centre Singapore (NHCS) started offering the continuous flow LVADs for patients on the transplant waiting list, and it was extended to non-transplant eligible patients (Destination therapy) in December 2012.
As of October 2017, the NHCS has implanted a total of 90 patients with excellent outcomes. Many of them are leading active working lives and can potentially turn up at the doorstep of any healthcare facility in Singapore. This article is a short guide for healthcare professionals, who might meet these patients.
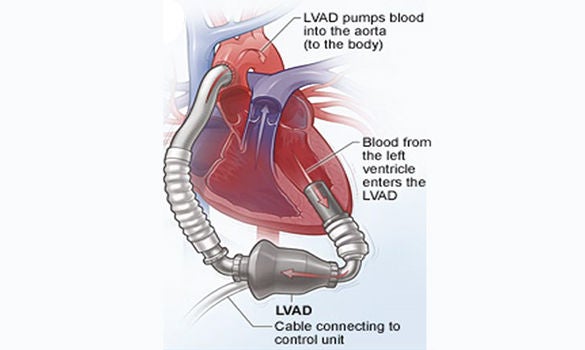
WHAT IS A LEFT VENTRICULAR ASSIST DEVICE (LVAD)?
The LVAD is an egg-sized rotary blood pump, that draws from the left ventricle and outputs into the ascending aorta. An electrical driveline exits from the upper abdomen, to obtain electrical power from a controller and 2 batteries. Over the past 8 years, we have used 3 different models of LVAD – the HeartMate II, HVAD and HeartMate III. (Refer to Figures 1, 2 and 3)

WHEN DO PATIENTS NEED AN LVAD?
The indications and contraindications for a surgery are summarised in Table 1. Patients should be referred before the low cardiac output state results in cachexia, as well as severe renal, liver, respiratory and other end-organ dysfunction, or even a circulatory collapse.
The risks for a surgery, and the chances of a good recovery, are much better before the patient requires dialysis, mechanical ventilation, an Intra-Aortic Balloon Pump (IABP) or Cardiopulmonary Resuscitation (CPR).
| Table 1 Surgery Indications and Contraindications | |
| Indications | Contraindications |
A combination of the following:
| Absolute
Relative
|
| *CRT indicates: Cardiac resynchronisation therapy DT: Destination therapy NYHA: New York Heart Association Vo2: Oxygen consumption PVD: Peripheral vascular disease | |
WHAT IS IT LIKE TO LIVE WITH AN LVAD?
The patients need to be on a long-term prescription for aspirin and warfarin. They must carry out daily dressing to the driveline. The batteries last up to 8 hours and they need to carry spare batteries if they go out. They can only shower with an adequate protection of the driveline exit site and the electrical equipment. They cannot swim.
The patients cope well with such inconveniences. They carry the battery and controller in inconspicuous backpacks, handbags, waist pouches or in custom-made vests. The device is so silent, that it becomes “forgettable” as the patients go about their daily lives.
The improvement in the effort tolerance is dramatic. The majority go from New York Heart Association (NYHA) Class 4 to Class 1 within a few months. Slightly under half of them go back to their work or studies. The rest of the patients do return to a normal active lifestyle.
Travelling overseas for the holidays is slightly inconvenient, with the need to carry the charger and extra batteries. However, this is manageable and not difficult with prior planning.
Sexual activity is not an issue. We strongly advise the younger female patients to use contraceptive measures, because a pregnancy will be hazardous.
WHAT DO HEALTHCARE PROVIDERS NEED TO KNOW ABOUT LVAD PATIENTS?
The most important thing that healthcare workers need to know, is that these patients have no pulse because the blood flows continuously, like water from a tap.
The aortic valve is closed almost all of the time. The LVAD will emit a continuous humming sound when you auscultate the heart, and the aortic and mitral heart sounds cannot be heard, only the softer pulmonary and tricuspid valves.
If the patient is unconscious, cardiac compressions should not be done if the pump is still operating well (humming is auscultated and there is no alarm from the controller), and the patient is pink, warm, and looks well perfused. There is a risk of pump dislodgement. Cardiac compressions should be started, if there are no signs of life, and there is clinically no perfusion of the body.
Alarms go off when the Ventricular Assist Device (VAD) malfunctions. There is a contact number on the controller for the NHCS VAD coordinators. One of our patients was saved by a passer-by at the Novena MRT station, when her pump stopped because of a controller failure. She became unconscious, the shrill VAD alarms went off, and her young daughter started screaming for help. A passer-by successfully changed her controller, with instructions from our VAD coordinator over a mobile phone.
The Blood Pressure (BP) is usually measured using a doppler probe and a pressure cuff. A pressure of 70 to 80 mmHg, at the point where the blood flow starts, is adequate. If an arterial line is used, a mean BP of 70 - 80 is also ideal.
If the patient is in Ventricular Tachycardia (VT) or Ventricular Fibrillation (VF), the cardioversion can be done without any damage to the pump. Surprisingly, most of the patients who develop VT or VF are usually only mildly symptomatic, and they remain conscious and ambulant because the pump flow remains adequate.
WHAT IS THE PROGNOSIS FOR PATIENTS SUPPORTED BY AN LVAD?
At NHCS, the perioperative mortality at 3 months is only 4.4%. 4 out of 5 patients are expected to survive for at least 4 years, compared with none before the LVADs were available.
Since May 2009 to September 2017, of the 89 patients we have seen, 47 are still on the LVAD support. Of these, 22 patients are on the transplant waiting list, 10 opted to remain on the LVAD and 15 were not suitable for a transplant, because of their age or co-morbidities. Eventually, 21 were transplanted, 2 recovered and 19 died.
The deaths occurred at various periods, from a few months to 4 years after the implant, due to complications that included strokes, pump thrombosis, a right ventricular failure, pump pocket infection, a bleeding gastrointestinal tract and kidney failure.
Other less serious complications include, a superficial driveline infection, depression and an equipment malfunction.
The patients’ average 6-minute walk test, at 6 months after surgery, is 437 metres, which is normal for any healthy individual. They also report large improvements in the quality of life after an LVAD implant.
SUMMARY
The LVADs are an excellent option for patients with an advanced heart failure. There are dramatic improvements in survival and in the quality of life. It has changed the lives of many all over the world. Most transplant programs all over the world, now perform more LVAD procedures than transplants.
Some patients have opted to remain on an LVAD, because they have not had any complications at all.
The average waiting time for a heart transplant in Singapore has increased from 153 days, before we had LVAD, to 680 days currently, because of the longer survival rate. The majority of the LVAD patients are likely to live with their LVAD for the rest of their lives.
GPs can call for appointments through the GP Appointment Hotline at 6704 2222 for more information.
For more interesting information, please visit the following websites:
HeartMate II
http://heartmateii.com/heart-failure-lvads.aspx
Youtube Video of an LVAD Patient
https://www.youtube.com/watch?v=gNUATS8Jhuk
A Blog Post by a Patient, Serene Lee, on Her Experience with the LVAD
https://paulineltl.wordpress.com/2015/05/03/keep-calm-mother-on-my-daughter-saved-my-life/amp
By:
Adjunct Assistant Professor Tan Teing Ee, Head and Senior Consultant,
Department of Cardiothoracic Surgery, and Co-Director, Mechanical Circulatory Support, Heart and Lung Transplant Unit,
National Heart Centre Singapore
Assistant Professor Tan Teing Ee is the Head and Senior Consultant with the Department of Cardiothoracic Surgery and the Director of the Cardiothoracic Surgery Intensive Care Unit at the National Heart Centre Singapore. He is also the Director of Quality Assurance and Risk Management, and the Co-Director, Heart Transplant and Mechanical Assist Device.
Assistant Professor Tan’s sub-specialty interest is in cardiac surgery (adult), robotics surgery, heart and lung transplant, and mechanical heart assist device. He performs adult cardiac surgeries, including coronary artery bypass, valvular heart disease, left ventricular assist device implantation and heart transplantation.














 Get it on Google Play
Get it on Google Play